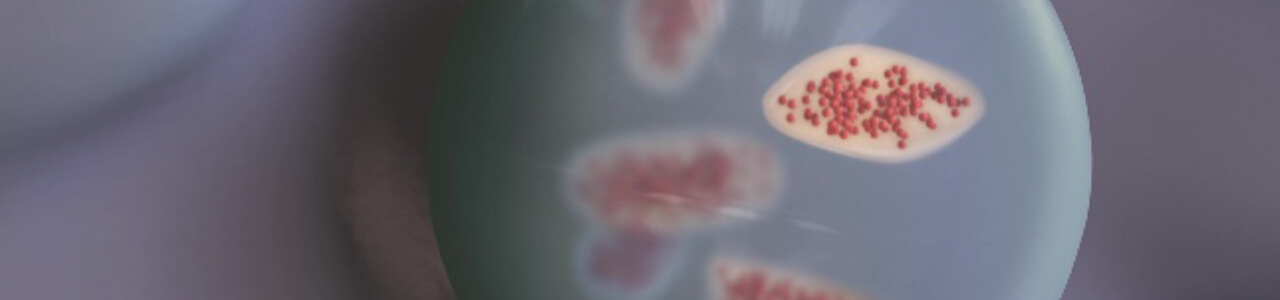
MECHANISM OF ACTION
MONOFERRIC DELIVERS A
SLOW AND CONTROLLED RELEASE
OF BIOAVAILABLE IRON1
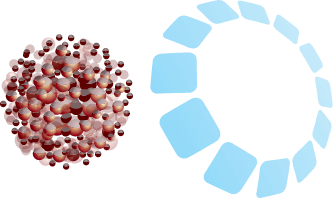
Components
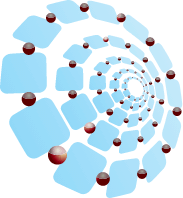
Delivery

Release
Artist rendition
- Serum ferritin levels peaked ~7 days after infusion, and slowly returned to stable levels at ~4 weeks2
- Tightly bound bioavailable iron slowly leaves the matrix to then bind to transferrin for incorporation into hemoglobin. RBC production follows2,3
INNOVATIVE MATRIX CONTROLS BIOAVAILABLE IRON RELEASE

INDICATIONS
Monoferric is indicated for the treatment of iron deficiency anemia (IDA) in adult patients:
- who have intolerance to oral iron or have had unsatisfactory response to oral iron
- who have non-hemodialysis dependent chronic kidney disease (NDD-CKD)
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Monoferric is contraindicated in patients with a history of serious hypersensitivity to Monoferric or any of its components. Reactions have included shock, clinically significant hypotension, loss of consciousness, and/or collapse.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Monoferric. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Monoferric administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Monoferric when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions. Monoferric is contraindicated in patients with prior serious hypersensitivity reactions to Monoferric or any of its components. In clinical trials in patients with IDA and CKD, serious or severe hypersensitivity were reported in 0.3% (6/2008) of the Monoferric treated subjects. These included 3 events of hypersensitivity in 3 patients; 2 events of infusion-related reactions in 2 patients and 1 event of asthma in one patient.
Iron Overload
Excessive therapy with parenteral iron can lead to excess iron storage and possibly iatrogenic hemosiderosis or hemochromatosis. Monitor the hematologic response (hemoglobin and hematocrit) and iron parameters (serum ferritin and transferrin saturation) during parenteral iron therapy. Do not administer Monoferric to patients with iron overload.
ADVERSE REACTIONS
Adverse reactions were reported in 8.6% (172/2008) of patients treated with Monoferric. Adverse reactions related to treatment and reported by ≥1% of the treated patients were nausea (1.2%) and rash (1%). Adjudicated serious or severe hypersensitivity reactions were reported in 6/2008 (0.3%) patients in the Monoferric group. Hypophosphatemia (serum phosphate <2.0 mg/dL) was reported in 3.5% of Monoferric-treated patients in Trials 1 & 2.
To report adverse events, please contact Pharmacosmos at 1-888-828-0655. You may also contact the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.
HAVE A QUESTION ABOUT HOW MONOFERRIC WORKS?
Sign up to contact a representative and receive information about Monoferric.
CONNECT WITH US


The safety and efficacy of Monoferric for treatment of iron deficiency anemia (IDA) were evaluated in two randomized, open-label, non-inferiority, actively controlled clinical trials performed in a total of 3050 patients with IDA of different etiologies.4,5
SEE THE RESULTS
1000 mg IV IRON REPLACEMENT IN A SINGLE INFUSION ≥20 MINUTES*
Monoferric offers iron replacement in a single 1000 mg dose for patients weighing 50 kg or more.2
SEE DOSING & ADMINISTRATION
*Repeat dose if iron deficiency anemia reoccurs.
MONOFERRIC
PATIENT PROFILES
Do you see any of these types of patients in your practice? See why Monoferric was right for them.
SEE THE PROFILES
INDICATIONS
Monoferric is indicated for the treatment of iron deficiency anemia (IDA) in adult patients:
- who have intolerance to oral iron or have had unsatisfactory response to oral iron
- who have non-hemodialysis dependent chronic kidney disease (NDD-CKD)
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Monoferric is contraindicated in patients with a history of serious hypersensitivity to Monoferric or any of its components. Reactions have included shock, clinically significant hypotension, loss of consciousness, and/or collapse.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Monoferric. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Monoferric administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Monoferric when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions. Monoferric is contraindicated in patients with prior serious hypersensitivity reactions to Monoferric or any of its components. In clinical trials in patients with IDA and CKD, serious or severe hypersensitivity were reported in 0.3% (6/2008) of the Monoferric treated subjects. These included 3 events of hypersensitivity in 3 patients; 2 events of infusion-related reactions in 2 patients and 1 event of asthma in one patient.
Iron Overload
Excessive therapy with parenteral iron can lead to excess iron storage and possibly iatrogenic hemosiderosis or hemochromatosis. Monitor the hematologic response (hemoglobin and hematocrit) and iron parameters (serum ferritin and transferrin saturation) during parenteral iron therapy. Do not administer Monoferric to patients with iron overload.
ADVERSE REACTIONS
Adverse reactions were reported in 8.6% (172/2008) of patients treated with Monoferric. Adverse reactions related to treatment and reported by ≥1% of the treated patients were nausea (1.2%) and rash (1%). Adjudicated serious or severe hypersensitivity reactions were reported in 6/2008 (0.3%) patients in the Monoferric group. Hypophosphatemia (serum phosphate <2.0 mg/dL) was reported in 3.5% of Monoferric-treated patients in Trials 1 & 2.
To report adverse events, please contact Pharmacosmos at 1-888-828-0655. You may also contact the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.
References:
- Kalra PA, Bhandari S. Int J Nephrol Renovasc Dis. 2016;9:53‐64.
- Monoferric (ferric derisomaltose) Prescribing Information; Pharmacosmos Therapeutics Inc., Morristown, NJ: 2022.
- Jahn MR, Andreasen HB, Fütterer S, et al. Eur J Pharm Biopharm. 2011;78(3):480‐491.
- Auerbach M, Henry D, Derman RJ, Achebe MM, Thomsen LL, Glaspy J. Am J Hematol. 2019;94(9):1007‐1014.
- Bhandari S, Kalra PA, Berkowitz M, Belo D, Thomsen LL, Wolf M. Nephrol Dial Transplant. 2021;36(1):111-120.
- Data on File. 2021. Pharmacosmos Therapeutics Inc.